A group of scientists investigated the impact of iridium and palladium nanoparticles on altering catalyst characteristics with minimal deterioration and transformation into an amorphous form. This collaborative effort was spearheaded by Skoltech Professor Alexander Kvashnin, along with members from Skoltech and Khakassian State University; Dr. Alexander Kvashnin holds a degree as a Doctor of Sciences in Physics and Mathematics.
The results are published in the Journal of Catalysis They offer understanding of key drivers for oxygen and hydrogen evolution reactions, along with oxygen reduction reactions.
Shifting from microparticles to nanoparticles causes significant alterations in the material’s physical and chemical characteristics. In nanoparticles, the quantity of atoms present on the surface is nearly identical to those within the particle itself.
Quantum effects, governed by the principles of quantum physics, significantly impact particle characteristics. These nanoparticles find applications virtually everywhere, including drug delivery mechanisms, LED displays, chemical fertilizers, and more.
"Palladium and iridium nanoalloys serve as vital catalysts for numerous organic reactions. Additionally, they play a key role in the oxidation process of carbon monoxide. Gaining insight into atomic-level processes is essential because determining the surface composition of such bimetallic nanoparticles proves challenging during actual experiments. It remains unclear whether they develop an outer layer, and if so, which kind offers superior stability in terms of energy consumption," noted Professor Alexander Kvashnin from the Skoltech Energy Transition Center.
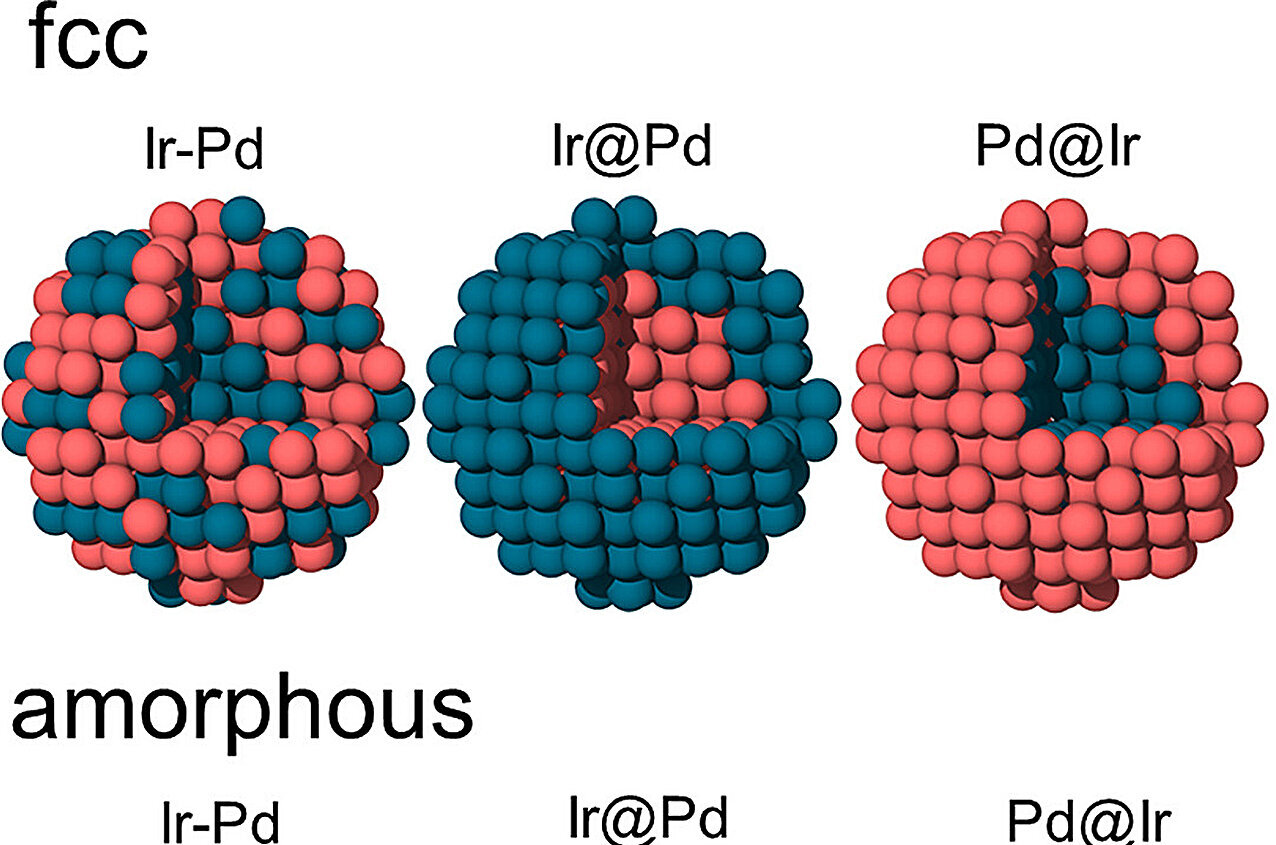
The geometric structure is essential to comprehending the catalytic properties of nanocatalysts. In the study, the authors examined core-shell iridium-palladium nanoparticles with different chemical ordering: iridium-core, palladium-shell and vice versa, as well as iridium and palladium alloys.
The research investigated the effect of the composition, type of structure (crystalline or amorphous), and local atomic environment of nanoparticles with a diameter of 2 nm on the electronic properties and charge distribution.
The melting point of nanoparticles is considerably lower compared to that of bulk palladium or iridium. Should the catalytic reactions occur at elevated temperatures, these particles might melt and convert into an amorphous form,” noted Ilya Chepkasov, who leads the study and serves as a senior researcher at the Skoltech Energy Transition Center.
A majority of studies concentrate on the characteristics of crystal structures. We aimed to observe how altering the particle’s state to amorphous might influence the properties of the catalyst.
The researchers determined that both the kind of core-shell nanoparticles and the thickness of the shell relative to the core significantly influence the surface charge.
Nanoparticles with an iridium center enveloped by a single-atom layer of palladium exhibit a notable surplus of electrons moving from the iridium core to the exterior, resulting in a negative charge at the surface. However, whether these nanoparticles have a crystalline or amorphous structure has almost no impact on their surface charge.
More information: Ilya V. Chepkasov and colleagues conducted a comprehensive first-principles investigation into the adsorption characteristics of both crystalline and amorphous PdIr nanoparticles. Journal of Catalysis (2025). DOI: 10.1016/j.jcat.2025.116102
Furnished by Skolkovo Institute of Science and Technology
The tale was initially released on Massima . Subscribe to our newsletter For the most recent science and technology news updates.