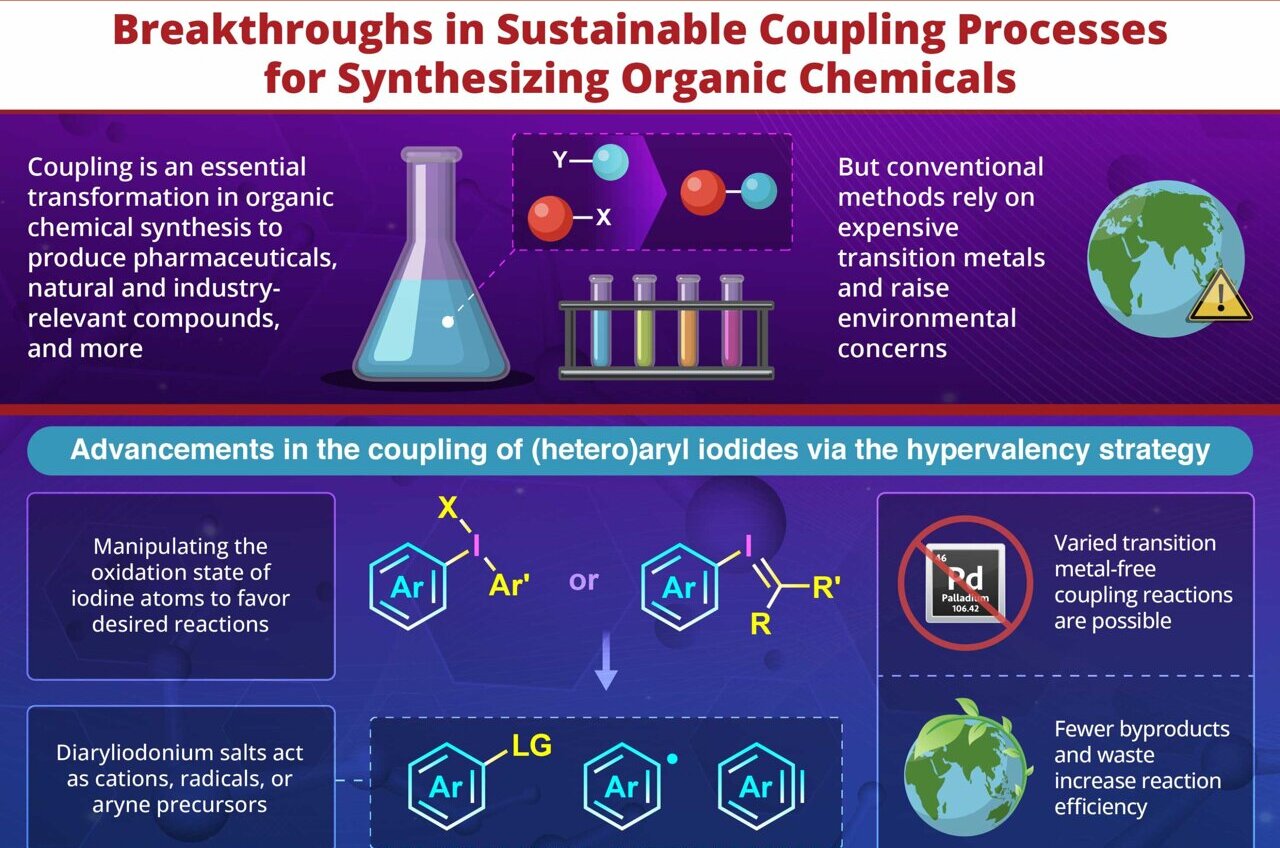
Coupling reactions stand out as some of the most revolutionary techniques in organic chemistry, facilitating the creation of essential chemical links found in drugs, agricultural chemicals, and cutting-edge materials. These processes have served as pillars of contemporary synthetic chemistry since their inception. Nonetheless, traditional approaches heavily depend on ecologically demanding transition-metal catalysts like palladium—substances that tend to be rare, expensive, and produce undesirable waste products.
Researchers are exploring new approaches due to the constraints imposed by traditional coupling techniques. These innovative strategies adhere to the tenets of green and sustainable chemistry (GSC), focusing on reducing waste production, decreasing dependence on scarce metallic elements, and cutting down on energy usage—all without compromising performance effectiveness or precision. Overcoming these hurdles is crucial for advancing greener practices in both industrial manufacturing processes and drug development protocols.
Currently, a research group headed by Professor Toshifumi Dohi from Ritsumeikan University’s College of Pharmaceutical Sciences and Professor Yasuyuki Kita, who serves as a Visiting Senior Researcher at Ritsumeikan University’s Research Organization of Science and Technology, offers an extensive summary of the latest developments in transition-metal-free coupling techniques. This review appears in a scholarly publication. Chemical Reviews On March 26, 2025, the focus is on highlighting new approaches that facilitate the triggering of aryl-iodide bonds within eco-friendly parameters.
The review notably highlights coupling through the hypervalent iodine approach, an area where the authors have been prominent figures for many years. The research group also comprised Dr. Elghareeb Elshahat Elboray, Dr. Kotaro Kikushima, and Dr. Koji Morimoto, who were affiliated with Ritsumeikan University.
The method utilizing hypervalent iodine relies on the distinctive characteristics of diaryliodonium salts, acting as very reactive intermediaries in coupling reactions. Through precise control over the oxidation states of iodine atoms, scientists can produce entities similar to aryl cations, radicals, and aryne precursors, aiding in targeted bond creation. This technique avoids the use of transition metals altogether, thereby decreasing dependence on expensive catalysts and improving the overall efficiency regarding atomic usage within these coupling procedures.
Prof. Dohi notes, "Our research introduces a hypervalency strategy—a cutting-edge advanced method designed to meet GSC standards more effectively—for coupling reactions aimed at synthesizing pharmaceuticals, associated molecules, and various functional organic compounds."
A major benefit of using hypervalent iodine-mediated coupling lies in its extensive range of substrates, enabling the effective creation of varied molecular structures. This technique further stands out due to its robustness with different functional groups, rendering it highly appealing for use in medicinal chemistry. Furthermore, scientists have developed several approaches to recover the aryl iodide by-products produced during these processes, thereby mitigating earlier worries over waste and improving the reaction’s atomic economy.
In addition to exploring hypervalent iodine techniques, the review delves into various transition-metal-free activation methodologies. These include base-facilitated aryl-iodide bond breaking, light-induced activation processes, electrochemical stimulation, and combined electro-photoactivation. Each method presents distinct advantages like lowering energy requirements, employing gentle reaction environments, or avoiding specific harmful substances. Through an examination of cutting-edge developments, the writers aim to encourage and motivate additional investigation within this field.
Prof. Kita states, 'We trust that this review will appeal to researchers seeking to create novel approaches for tackling the challenges linked to this area of chemistry.'
As the demand increases for environmentally friendly and cost-effective approaches to chemical synthesis, the methodologies discussed in this overview could redefine the landscape of organic chemistry. These techniques aim to lessen ecological footprints and decrease manufacturing expenses, potentially making significant strides in the sustained growth of drugs and various specialty chemicals. With ongoing advancements in the discipline, it’s hoped that today's insights can lay down essential groundwork for forthcoming innovations within the sphere of green chemistry.
More information: Toshifumi Dohi et al., Activating Iodoarenes: A Step Ahead Towards Greener and More Sustainable Conversions Chemical Reviews (2025). DOI: 10.1021/acs.chemrev.4c00808
Provided by Ritsumeikan University
This tale was initially released on Massima . Subscribe to our newsletter For the most recent updates on science and technology news.