Research teams from South Korea have introduced a new method for cancer immunotherapy that might be used alongside current CAR-T (Chimeric Antigen Receptor T-cell) therapies. This research proposes an innovative addition. published in the journal Biomarker Research .
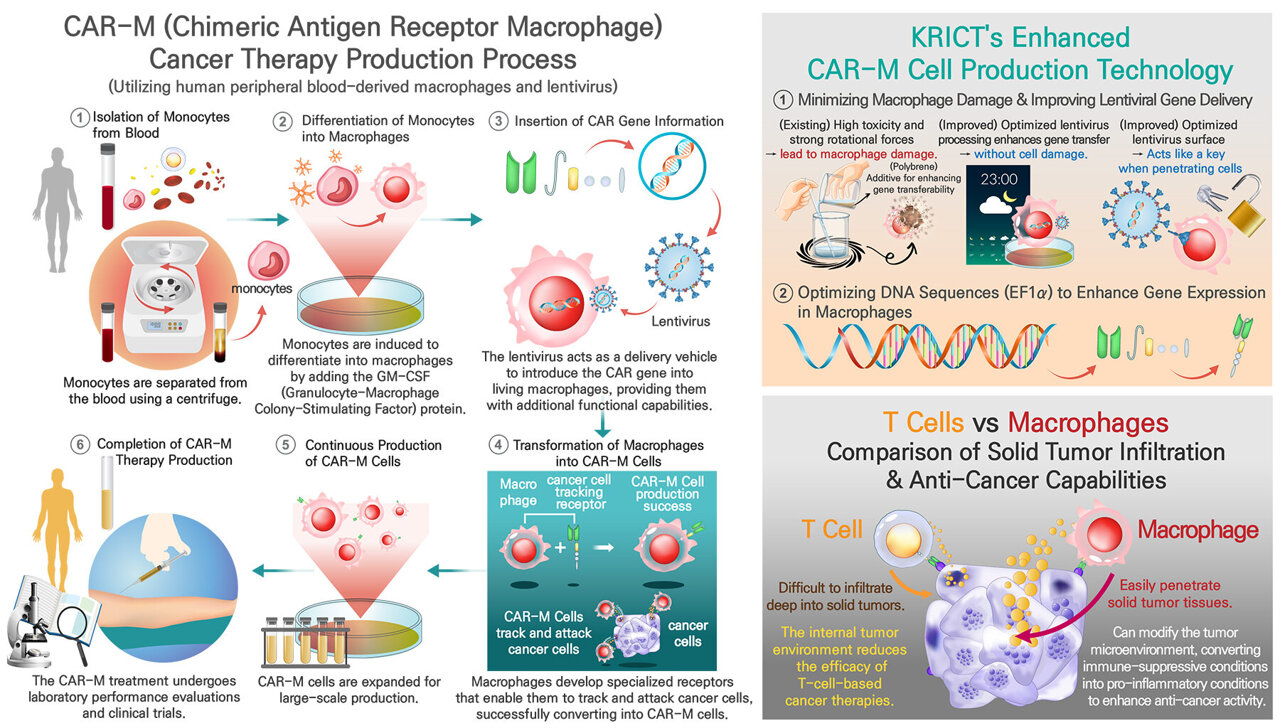
Scientists at the Korea Research Institute of Chemical Technology (KRICT), under the leadership of Dr. Chi Hoon Park, have managed to create Chimeric Antigen Receptor Macrophages (CAR-M) by effectively incorporating artificial genes into human macrophages obtained from peripheral blood through the use of a lentiviral delivery method. This advancement is anticipated to broaden the scope of CAR therapies from just treating blood cancers to also addressing solid tumors.
CAR-T cell therapy entails removing a patient’s T cells, altering their genetic makeup so they can attack particular cancer cells, then putting these modified cells back into the individual. Although this approach has shown remarkable success with hematological malignancies including leukemia, it encounters substantial hurdles when applied to solid tumors like those found in lung cancer.
Macrophages, a kind of immune cell, can penetrate solid tumors more efficiently compared to T cells, making them a promising option for cancer treatment. Nonetheless, current treatments based on macrophages have faced challenges, mainly because the genetic alterations do not last long, thereby decreasing their effectiveness as a therapy.
The research group created several cutting-edge methods for efficiently introducing synthetic genes that code for CAR into macrophages without inducing cell harm.
- Removing toxicity: Traditional gene transfer techniques employ polybrene, an positively charged polymer that boosts viral entry yet proves extremely harmful to macrophages. To address this issue, the scientists removed polybrene and increased the duration of viral contact from 1.5 hours to 16 hours, thereby achieving a safer and more effective method for gene delivery.
- Improving Transduction Timing: The research showed that the effectiveness of gene absorption in macrophages varies according to their developmental phase. Postponing the infection till day seven of differentiation notably enhanced the levels of gene expression.
- Improving viral entry: The group refined the VSV-G (Vesicular Stomatis Viru sG) protein, which plays a crucial role in facilitating viruses' access to host cells, through alterations in its codon composition. These changes notably boosted the effectiveness of gene delivery.
- To maintain consistent gene expression, the scientists used the EF1α promoter, enabling macrophages to keep the CAR gene active for as long as 20 days, thus addressing past issues with gene instability.
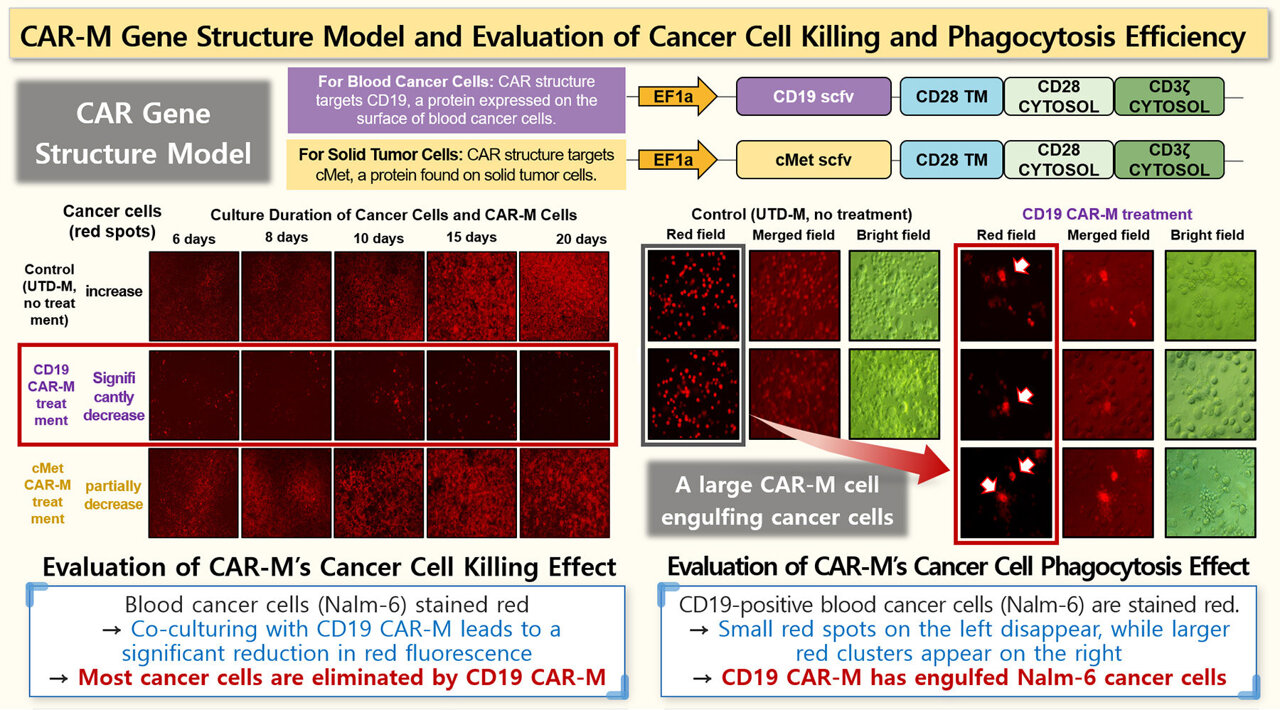
The CAR-M cells produced following this procedure exhibited significant anti-tumor activity. Upon being cultured alongside Nalm6 (acute lymphoblastic leukemia) and Raji (B-cell lymphoma) cells, these CAR-M macrophages successfully ingested and eliminated the cancerous cells, as confirmed via fluorescence microscopy observations.
The research group intends to increase the manufacturing of CAR-M and refine high-efficiency treatment procedures for use in clinical settings.
Dr. Chi Hoon Park remarked, "This research marks the first time stable CAR expression has been achieved in macrophages obtained from peripheral blood through the use of lentivirus."
The president of KRICT, Young-Kuk Lee, highlighted that "this technology has the potential to enhance current CAR-T therapies and help broaden the range of options in immuno-oncology treatments."
More information: Ji U Choi et al., Production of Human Chimeric Antigen Receptor Macrophages from Peripheral Blood Using Lentivirus-Based Methods, Biomarker Research (2025). DOI: 10.1186/s40364-024-00703-9
Presented by the National Research Council of Science and Technology
This tale was initially released on Medical Xpress . Subscribe to our newsletter For the most recent updates on science and technology.